Seafloor massive sulfide (SMS) deposits are formed when boiling, mineral-rich hydrothermal fluids vent on the seabed, a process that usually occurs at spreading ridges.
These sulfide deposits often contain substantial amounts of valuable metals, including copper, nickel, zinc, gold, and silver. Exploration companies target inactive vents to avoid the challenges posed by high-temperature fluids and to minimize disturbance to vulnerable marine fauna.
However, as extinct SMS deposits age and interact with seawater, their composition changes—sometimes for the better and sometimes for the worse.
How metals behave in aging sulfide deposits remains poorly understood.
At the Deep Sea Minerals conference in Bergen in April, University of Southampton researcher Christian Star Bishop will share some insights into his team’s investigation at the Semenov hydrothermal field in the Atlantic Ocean. Semenov is considered to host the largest known sulfide deposits.
The project participants conducted two research expeditions to the Mid-Atlantic spreading ridge in 2022 and 2023 and analyzed the collected samples in order to find out what happens to the sulfide metals over time.
When sulfide minerals such as pyrite and chalcopyrite are exposed to oxygen and seawater, they break down into new minerals. These new minerals, including atacamite and iron oxyhydroxide (Fe-oxyhydroxide), act like sponges, absorbing copper and zinc.
Atacamite collected at the site contained an average of 2.87 wt.% copper, while Fe-oxyhydroxide held 1.38 wt.%.
The lab studies revealed that the type of sulfide material influences the retention of metals. Specifically, Fe-oxyhydroxide derived from chalcopyrite retains more copper, while pyrite-derived forms tend to hold more zinc.
However, as sulfide deposits age and undergo further oxidation, they appear to lose a significant amount of their copper and zinc. Fe-oxyhydroxide precipitates as ferrihydrite, which can later crystallize into goethite. This transformation results in the release of approximately 92% of the copper and 57% of the zinc originally contained in the Fe-oxyhydroxide.
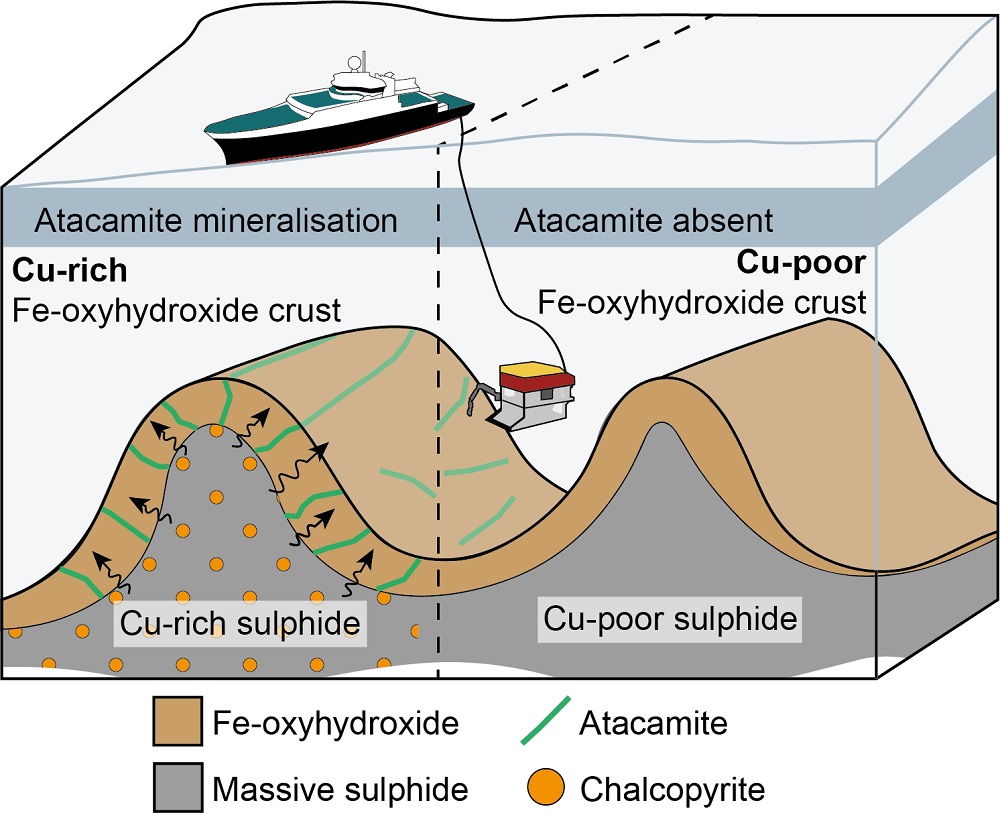
As a result, it is likely that older, off-axis deposits are considerably depleted of these metals due to the prolonged processes of oxidation and goethite formation.
SMS deposits may also be rich in gold and silver. The project results indicate that the precious metals behave differently during weathering. Instead of being absorbed into Fe-oxyhydroxide, they are either lost in seawater or concentrated in specific zones known as redox fronts at the margins of sulfide deposits.
This suggests that areas with enriched gold and silver within partially weathered sulfide could serve as promising targets for future exploration.
The results from the study may help to guide future exploration of SMS deposits through an enhanced understanding of the mobility and behavior of metals. Fe-oxyhydroxide and atacamite could serve as valuable clues, highlighting areas with the greatest copper concentration in sulfide weathering products and underlying massive sulfides.
Deep Sea Minerals 2025 will take place in Bergen from 1-3 April. The program and registration page can be found on the conference website.

![var rnd = window.rnd || Math.floor(Math.random()*10e6);
var pid980600 = window.pid980600 || rnd;
var plc980600 = window.plc980600 || 0;
var abkw = window.abkw || '';
var absrc = 'https://servedbyadbutler.com/adserve/;ID=188736;size=0x0;setID=980600;type=js;sw='+screen.width+';sh='+screen.height+';spr='+window.devicePixelRatio+';kw='+abkw+';pid='+pid980600+';place='+(plc980600++)+';rnd='+rnd+'';
var _absrc = absrc.split("type=js"); absrc = _absrc[0] + 'type=js;referrer=' + encodeURIComponent(document.location.href) + _absrc[1];
document.write('');
Understanding the fate of sulfide metals](https://geo365.no/wp-content/uploads/2025/03/1000_Sulfide-project-ULTRA.jpg)